To study production of an x-ray fluorescence spectrum in a solid sample with primary x-rays, emitted by an anode of the x-ray tube. To study physical processes in a Si-PIN photodiode used as a detector of the x-ray spectrum. To study functioning of a multichannel analyzer of the x-ray spectrum and spectra processing.
- Wavelength Dispersive X Ray Spectroscopy
- X-ray Texture Pack 1.16.1
- Energy Dispersive Spectroscopy Basics
- X-ray Texture Pack 1.15.2
- X-ray Minecraft Texture Pack
- Energy-dispersive X-ray spectroscopy (EDS) mapping has been performed to determine the distributions of the component atoms of η-Mg(Zn,Cu) 2 precipitates in an Al-Mg-Zn-Cu aluminium alloy. High-angle annular dark-field scanning transmission electron microscopy (HAADF STEM) was concomitantly employed to recognize the configurations of atomic.
- Energy-dispersive X-ray spectroscopy (also known as EDS, EDX, or EDXA) is a powerful technique that enables the user to analyze the elemental composition of a desired sample. The major operating principle that allows EDS to function is the capacity of high energy electromagnetic radiation (X-rays) to eject 'core' electrons (electrons that are.
- ENERGY DISPERSIVE X-RAY SPECTROSCOPY (EDS) Energy Dispersive X-Ray Spectroscopy (EDS) is an analytical technique to qualitatively and quantitatively identify the elemental composition of materials analyzed in an SEM. EDS analyzes the top few microns of the sample with a spatial resolution as small as one micron.
- Energy-dispersive X-ray spectroscopy (EDX or EDS) is an analytical technique used to probe the composition of a solid materials. Several variants exist, but the all rely on exciting electrons near the nucleus, causing more distant electrons to drop energy levels to fill the resulting “holes.”.
Energy-dispersive X-ray spectroscopy (EDS, EDX, EDXS or XEDS), sometimes called energy dispersive X-ray analysis (EDXA) or energy dispersive X-ray microanalysis (EDXMA), is an analytical technique used for the elemental analysis or chemical characterization of a sample. It relies on an interaction of some source of X-ray excitation and a sample. Its characterization capabilities are due in large part to the fundamental principle that each element has a unique atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum[2] (which is the main principle of spectroscopy). The peak positions are predicted by the Moseley's law with accuracy much better than experimental resolution of a typical EDX instrument.
To stimulate the emission of characteristic X-rays from a specimen a beam of X-rays is focused into the sample being studied. At rest, an atom within the sample contains ground state (or unexcited) electrons in discrete energy levels or electron shells bound to the nucleus. The incident beam may excite an electron in an inner shell, ejecting it from the shell while creating an electron hole where the electron was. An electron from an outer, higher-energy shell then fills the hole, and the difference in energy between the higher-energy shell and the lower energy shell may be released in the form of an X-ray. The number and energy of the X-rays emitted from a specimen can be measured by an energy-dispersive spectrometer. As the energies of the X-rays are characteristic of the difference in energy between the two shells and of the atomic structure of the emitting element, EDS allows the elemental composition of the specimen to be measured.[2]

Equipment[edit]
Four primary components of the EDS setup are
- the excitation source (electron beam or x-ray beam)
- the X-ray detector
- the pulse processor
- the analyzer.[citation needed]
Electron beam excitation is used in electron microscopes, scanning electron microscopes (SEM) and scanning transmission electron microscopes (STEM). X-ray beam excitation is used in X-ray fluorescence (XRF) spectrometers. A detector is used to convert X-ray energy into voltage signals; this information is sent to a pulse processor, which measures the signals and passes them onto an analyzer for data display and analysis.[citation needed] The most common detector used to be Si(Li) detector cooled to cryogenic temperatures with liquid nitrogen. Now, newer systems are often equipped with silicon drift detectors (SDD) with Peltier cooling systems.
Technological variants[edit]
The excess energy of the electron that migrates to an inner shell to fill the newly created hole can do more than emit an X-ray.[3] Often, instead of X-ray emission, the excess energy is transferred to a third electron from a further outer shell, prompting its ejection. This ejected species is called an Auger electron, and the method for its analysis is known as Auger electron spectroscopy (AES).[3]
X-ray photoelectron spectroscopy (XPS) is another close relative of EDS, utilizing ejected electrons in a manner similar to that of AES. Information on the quantity and kinetic energy of ejected electrons is used to determine the binding energy of these now-liberated electrons, which is element-specific and allows chemical characterization of a sample.[citation needed]
EDS is often contrasted with its spectroscopic counterpart, WDS (wavelength dispersive X-ray spectroscopy). WDS differs from EDS in that it uses the diffraction of X-rays on special crystals to separate its raw data into spectral components (wavelengths). WDS has a much finer spectral resolution than EDS. WDS also avoids the problems associated with artifacts in EDS (false peaks, noise from the amplifiers, and microphonics).
A high-energy beam of charged particles such as electrons or protons can be used to excite a sample rather than X-rays. This is called Particle-induced X-ray Emission) or PIXE.
Accuracy of EDS[edit]
EDS can be used to determine which chemical elements are present in a sample, and can be used to estimate their relative abundance. EDS also helps to measure multi-layer coating thickness of metallic coatings and analysis of various alloys. The accuracy of this quantitative analysis of sample composition is affected by various factors. Many elements will have overlapping X-ray emission peaks (e.g., Ti Kβ and V Kα, Mn Kβ and Fe Kα). The accuracy of the measured composition is also affected by the nature of the sample. X-rays are generated by any atom in the sample that is sufficiently excited by the incoming beam. These X-rays are emitted in all directions (isotropically), and so they may not all escape the sample. The likelihood of an X-ray escaping the specimen, and thus being available to detect and measure, depends on the energy of the X-ray and the composition, amount, and density of material it has to pass through to reach the detector. Because of this X-ray absorption effect and similar effects, accurate estimation of the sample composition from the measured X-ray emission spectrum requires the application of quantitative correction procedures, which are sometimes referred to as matrix corrections.[2]
Emerging technology[edit]
There is a trend towards a newer EDS detector, called the silicon drift detector (SDD). The SDD consists of a high-resistivity silicon chip where electrons are driven to a small collecting anode. The advantage lies in the extremely low capacitance of this anode, thereby utilizing shorter processing times and allowing very high throughput. Benefits of the SDD include:[citation needed]
- High count rates and processing,
- Better resolution than traditional Si(Li) detectors at high count rates,
- Lower dead time (time spent on processing X-ray event),
- Faster analytical capabilities and more precise X-ray maps or particle data collected in seconds,
- Ability to be stored and operated at relatively high temperatures, eliminating the need for liquid nitrogen cooling.
Because the capacitance of the SDD chip is independent of the active area of the detector, much larger SDD chips can be utilized (40 mm2 or more). This allows for even higher count rate collection. Further benefits of large area chips include:[citation needed]
- Minimizing SEM beam current allowing for optimization of imaging under analytical conditions,
- Reduced sample damage and
- Smaller beam interaction and improved spatial resolution for high speed maps.
Wavelength Dispersive X Ray Spectroscopy
Where the X-ray energies of interest are in excess of ~ 30 keV, traditional silicon-based technologies suffer from poor quantum efficiency due to a reduction in the detector stopping power. Detectors produced from high density semiconductors such as cadmium telluride (CdTe) and cadmium zinc telluride (CdZnTe) have improved efficiency at higher X-ray energies and are capable of room temperature operation. Single element systems, and more recently pixelated imaging detectors such as the HEXITEC system, are capable of achieving energy resolutions of the order of 1% at 100 keV.
In recent years, a different type of EDS detector, based upon a superconducting microcalorimeter, has also become commercially available. This new technology combines the simultaneous detection capabilities of EDS with the high spectral resolution of WDS. The EDS microcalorimeter consists of two components: an absorber, and a superconducting transition-edge sensor (TES) thermometer. The former absorbs X-rays emitted from the sample and converts this energy into heat; the latter measures the subsequent change in temperature due to the influx of heat. The EDS microcalorimeter has historically suffered from a number of drawbacks, including low count rates and small detector areas. The count rate is hampered by its reliance on the time constant of the calorimeter's electrical circuit. The detector area must be small in order to keep the heat capacity small and maximize thermal sensitivity (resolution). However, the count rate and detector area have been improved by the implementation of arrays of hundreds of superconducting EDS microcalorimeters, and the importance of this technology is growing.
See also[edit]
References[edit]
- ^Corbari, L; et al. (2008). 'Iron oxide deposits associated with the ectosymbiotic bacteria in the hydrothermal vent shrimp Rimicaris exoculata'(PDF). Biogeosciences. 5 (5): 1295–1310. doi:10.5194/bg-5-1295-2008.
- ^ abcJoseph Goldstein (2003). Scanning Electron Microscopy and X-Ray Microanalysis. Springer. ISBN978-0-306-47292-3. Retrieved 26 May 2012.
- ^ abJenkins, R. A.; De Vries, J. L. (1982). Practical X-Ray Spectrometry. Springer. ISBN978-1-468-46282-1.
External links[edit]
- MICROANALYST.NET – Information portal with X-ray microanalysis and EDX contents
- [1] -EDS on the SEM: Primer discussing principles, capabilities and limitations of EDS with the SEM
- Learn how to do EDS in an SEM – an interactive learning environment provided by Microscopy Australia
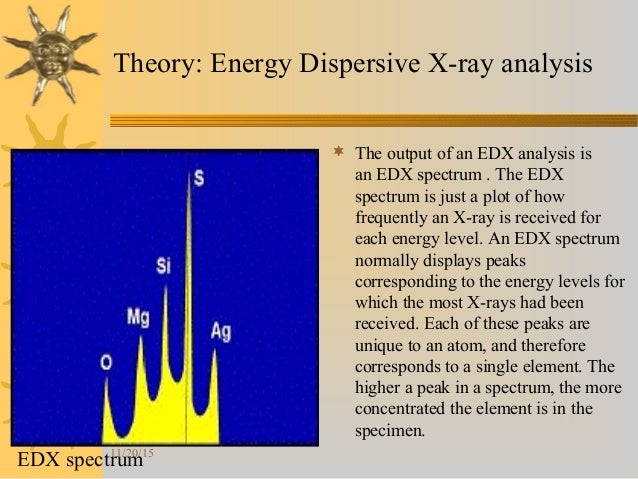
Energy Dispersive X-ray Spectroscopy (EDS or EDX) is a qualitative and quantitative X-ray microanalytical technique that provides information on the chemical composition of a sample for elements with atomic number (Z) >3.
Characteristic X-ray Generation
The atoms are ionized by the primary electron beam leading to holes generated on the core shells; following ionization the electrons from outer shells fill the holes and cause the emission of X-ray fluorescence lines.
The characteristic X-ray lines are named according to the shell in which the initial vacancy occurs and the shell from which an electron drops to fill that vacancy.
.jpg)

For instance, if the initial vacancy occurs in the K shell and the vacancy filling electron drops from the adjacent (L) shell, a Kα x-ray is emitted. If the electron drops from the M shell (two shells away), the emitted x-ray is a Kβ x-ray. Similarly, if an L-shell electron is ejected and an electron from the M-shell fills the vacancy, Lα radiation will be emitted.
EDS Detector
The detector is based on a semiconductor device, usually a crystal of silicon. The first detector developed was the lithium-drifted silicon or Si(Li) detector, which is now giving way to the silicon-drift detector or SDD.
A typical EDS detector is composed of
X-ray Texture Pack 1.16.1

Energy Dispersive Spectroscopy Basics
- A collimator to ensure that only X-rays generated from where the primary electron beam interacts with the sample will be collected.
- An electron trap to ensure that X-rays, but no electrons, enter the detector.
A window to isolate the detector crystal, under high vacuum, from the chamber of the microscope. Older windows were composed of Be which did not allow low-energy X-rays (< ~0.9 keV) to pass through it, but more modern windows are composed of polymers that will allow low-energy X-rays (down to ~0.1 keV) to pass.
A semiconductor crystal detector.
Electronics to detect the charge recorded by the detector, convert it to a voltage pulse and pass it to the pulse processor.
Detector Operating Principle
X-ray Texture Pack 1.15.2
- The energy of the incoming X-ray is dissipated by the creation of a series of electron-hole pairs in the semiconductor crystal.
- A high bias voltage is applied across the crystal and this causes electrons and holes to move to electrodes on opposite sides of the crystal, producing a charge signal which is passed to the pulse processor.
- The size of the signal is proportional to the energy of the incoming X-ray. For a silicon detector, ~3.8 eV is used to generate each electron-hole pair (~2.9 eV for Ge). So for an incoming Ni Kα X-ray of energy 7.477 keV, 1968 electron-hole pairs will be produced, and for an Al Kα X-ray of 1.487 keV, 391 electron-hole pairs will be generated.
- By measuring the amount of current produced by each X-ray photon, the original energy of the X-ray can be calculated. An EDS spectrum is essentially a histogram of the number of X-rays measured at each energy.